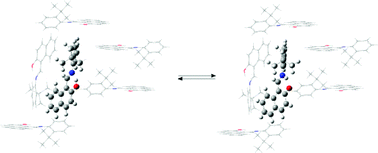
Schiff bases derived from hydroxyl naphthaldehydes and o-substituted anilines have been prepared and their tautomerism assessed by spectroscopic, crystallographic, and computational methods. Tautomeric equilibria have also been studied and reveal in most cases a slight preference of imine tautomers in solution; a fact supported by DFT calculations in the gas phase as well as incorporating solvent effects through the SMD model. To simulate the effect exerted by the crystal lattice on tautomer stability, we have developed a computational protocol in the case of 1-tert-butyl-2-(2-hydroxy-1-naphthylmethylene)aminobenzene whose data have been obtained experimentally at 120 K. Although a rapid imine-enamine interconversion may be occurring in the solid state, the imine tautomer becomes the most stable form and the energy difference should be related to the difference in the packing of the molecules.