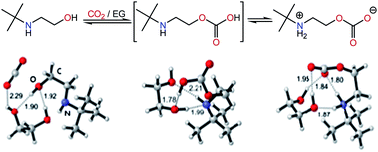
Sterically hindered amines such as 2-[(1,1-dimethylethyl)amino]ethanol (TBAE) and 1-[(1,1-dimethylethyl)amino]-2-propanol (TBAP) were found to reversibly interact with CO2 in a 1 : 1 molar ratio exclusively through the hydroxyl group, producing zwitterionic carbonate species, which lose CO2 at considerably lower temperatures than the CO2-adducts of other alkanolamines including monoethanolamine and diethanolamine. The formation of zwitterionic carbonate species from TBAE and TBAP was supported by spectroscopic and computational studies.