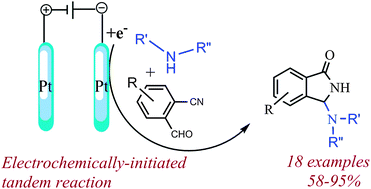
N-Mannich bases of 1-isoindolinones can be rapidly assembled by reacting 2-formylbenzonitriles with electroactivated amines on a Pt cathode, using a catalytic amount of electricity. Usefully, chiral amines allow the attainment of enantiopure N-Mannich bases by simple chromatographic separation.