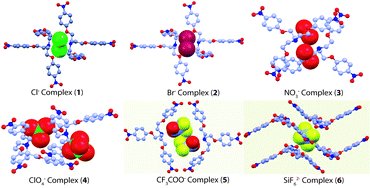
The structural aspects of binding of halides (1 and 2), nitrate (3), perchlorate (4), trifluoroacetate (5) and hexafluorosilicate (6) with the protonated tripodal podand L are examined crystallographically. Anion binding with multiple receptor units is attributable entirely to the (NH)+⋯anion and multiple C–H⋯anion hydrogen bonding interactions in all six complexes. Protonation at the apical nitrogen and presence of nitro functionality renders the methylene and aryl hydrogen sufficiently acidic for their active participation in moderate to weak CH⋯anion interactions are noteworthy. All supramolecular networks of complexes 1–6 are guided by various non-convalent interactions, especially directional CH⋯Onitro hydrogen bonds and π-stacking interactions.