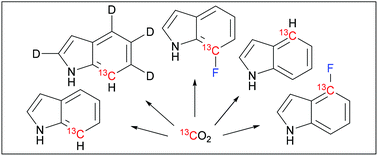
Synthesis of indoles labeled with 13C–1H and 13C–19F spin pairs is described. All syntheses utilize inexpensive carbon–13C dioxide as the 13C isotope source. Ruthenium-mediated ring-closing metathesis is the key step in construction of the 13C containing indole carbocycle. Fluorine is introduced via electrophilic fluorination at the 7-position and via palladium-mediated cross-coupling at the 4-position. Indole and fluoroindoles are viable tryptophan precursors for in vivo protein expression. We show that they are viable also in in vitro protein synthesis using standard E. coli S30 extracts. Incorporation of the synthesized 13C–1H and 13C–19F spin pair labeled tryptophans into proteins enables high-resolution and high-sensitivity nuclear magnetic resonance (NMR) spectroscopy.