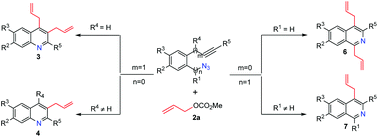
A novel and efficient strategy for one-step synthesis of allylated quinolines and isoquinolines via palladium-catalyzed cyclization–allylation of azides and allyl methyl carbonate is developed for the first time. The results indicated that the regioselective synthesis of allyl- and diallyl-substituted quinolines/isoquinolines depends on different substituted groups at R1 and R4 positions, such as H or other groups. The reactions proceed smoothly in the presence of Pd(PPh3)4 and K3PO4 or NaOAc in DMF at 100 °C to give the corresponding allyl- and diallyl-substituted quinolines and isoquinolines in good to high yields.