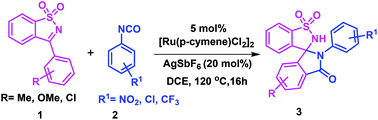
A novel one-pot strategy has been developed for the construction of spiro-isoindolinone-benzosultams through a Ru(II)-catalyzed sequential C–C and C–N bond formation. This method is highly atom-economical and compatible with a wide range of substituents. This is the first report on the spirocyclization of 3-aryl N-sulfonyl ketimines with aryl isocyanates to produce highly rigid and pharmaceutically relevant spirocycles through a C–H bond activation.