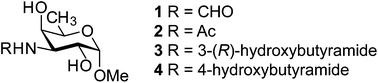Bacterial polysaccharides may contain rare sugars of different stereochemistry and diverse functional groups; the repertoire can be further extended by varying the exocyclic substituents. Synthesis of four monosaccharides is described utilizing a suitably protected key intermediate obtained by regioselective acetal ring-opening reduction, dexoygenation at C6, alcohol oxidation at C3 followed by formation of an oxime, which was stereoselectively reduced by samarium diiodide to give a 3-amino-derivative having the desired galacto-configuration. Subsequent functionalization was performed resulting in one to four carbon atoms in the amide substituent.