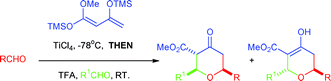
A one-pot, multi-component reaction for the synthesis of highly substituted tetrahydropyran-4-ones, based on the long forgotten Maitland–Japp reaction has been realised. Two different aldehydes and a derivative of a β-ketoester can be condensed regioselectively in the presence of a Lewis acid to form tetrahydropyran-4-ones in excellent yields. The diastereoselectively of the reaction was found to be dependant upon the nature of the Lewis acid and the temperature at which the reaction was carried out. This procedure was also extended to the formation of tetrahydropyran-4-ones in greater than 95% enantiomeric excess.