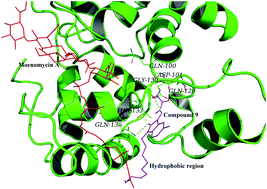
A series of isatin derivatives bearing three different substituent groups at the N-1, C-3 and C-5 positions of the isatin scaffold were systematically designed and synthesized to study the structure–activity relationship of their inhibition of bacterial peptidoglycan glycosyltransferase (PGT) activity and antimicrobial susceptibility against S. aureus, E. coli and methicillin-resistant Staphylococcus aureus (MRSA (BAA41)) strains. The substituents at these sites are pointing towards three different directions from the isatin scaffold to interact with the amino acid residues in the binding pocket of PGT. Comparative studies of their structure–activity relationship allow us to gain better understanding of the direction of the substituents that contribute critical interactions leading to inhibition activity against the bacterial enzyme. Our results indicate that the modification of these sites is able to maximize the antimicrobial potency and inhibitory action against the bacterial enzyme. Two compounds show good antimicrobial potency (MIC = 3 μg mL−1 against S. aureus and MRSA; 12–24 μg mL−1 against E. coli). Results of the inhibition study against the bacterial enzyme (E. coli PBP 1b) reveal that some compounds are able to achieve excellent in vitro inhibitions of bacterial enzymatic activity (up to 100%). The best half maximal inhibitory concentration (IC50) observed among the new compounds is 8.9 μM.