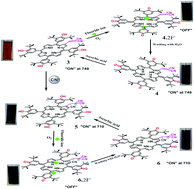
β-Functionalization of meso-tetrakis(3,5-di-tert-butyl-4-hydroxyphenyl)porphyrin with electron acceptors such as formyl and dicyanovinyl has been reported for the first time. 2-Formyl-5,10,15,20-tetrakis(3′,5′-di-tert-butyl-4′-hydroxyphenyl)porphyrinatocopper(II) (Cu-TDtBHPP-CHO) crystallizes in the triclinic space group P![[1 with combining macron]](http://www.rsc.org/images/entities/char_0031_0304.gif) , [a = 10.8479(4) Å, b = 14.6207(5) Å, c = 15.9745(5) Å, V = 2198.97(13) Å3] and exhibits an almost planar structure and a square planar geometry. β-Formyl/dicyanovinyl substituted porphyrins such as Cu-TDtBHPP-CHO, Ni-TDtBHPP-CHO, Cu-TDtBHPP-MN (1), Ni-TDtBHPP-MN (2) and H2-TDtBHPP-MN (3) exhibited red-shifted optical absorption features (Δλmax = 13–40 nm) in CH2Cl2 compared to the corresponding MTPPs. β-Dicyanovinyl substituted porphyrins were developed as a quantitatively operating ‘lab-on-a-molecule’ for the visual detection of F− and CN− ions. Having a CN− ion responsive dicyanovinyl moiety and a F− ion responsive redox-active 3,5-di-tert-butyl-4-hydroxyphenyl groups, they detect F− and CN− ions simultaneously by switching unique structural changes between porphyrin, porphodimethene and porphyrinogen along with distinct colour changes which were monitored by UV-Vis-NIR, fluorescence and NMR spectroscopic techniques.
, [a = 10.8479(4) Å, b = 14.6207(5) Å, c = 15.9745(5) Å, V = 2198.97(13) Å3] and exhibits an almost planar structure and a square planar geometry. β-Formyl/dicyanovinyl substituted porphyrins such as Cu-TDtBHPP-CHO, Ni-TDtBHPP-CHO, Cu-TDtBHPP-MN (1), Ni-TDtBHPP-MN (2) and H2-TDtBHPP-MN (3) exhibited red-shifted optical absorption features (Δλmax = 13–40 nm) in CH2Cl2 compared to the corresponding MTPPs. β-Dicyanovinyl substituted porphyrins were developed as a quantitatively operating ‘lab-on-a-molecule’ for the visual detection of F− and CN− ions. Having a CN− ion responsive dicyanovinyl moiety and a F− ion responsive redox-active 3,5-di-tert-butyl-4-hydroxyphenyl groups, they detect F− and CN− ions simultaneously by switching unique structural changes between porphyrin, porphodimethene and porphyrinogen along with distinct colour changes which were monitored by UV-Vis-NIR, fluorescence and NMR spectroscopic techniques.