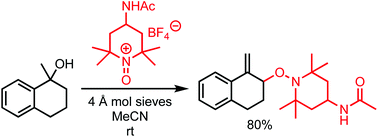
Tertiary benzylic alcohols react with oxoammonium salts, undergoing a tandem elimination/allylic oxidation to provide an allylic ether product in a single step. This mode of reactivity provides a rapid entry into allylic ethers from certain benzylic tertiary alcohols. The allylic ether may be cleaved under reductive conditions to reveal the allylic alcohol.