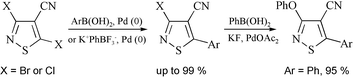
3,5-Dichloroisothiazole-4-carbonitrile 1 reacts with aryl- and methylboronic acids to give in high yields the 3-chloro-5-(aryl and methyl)-isothiazole-4-carbonitrile 2 regiospecifically. The reaction was optimized with respect to base, phase transfer agent and palladium catalyst. Suzuki coupling at C-5 was also achieved in high yield using potassium phenyltrifluoroborate. The regiospecificity of either coupling method is maintained with 3,5-dibromoisothiazole-4-carbonitrile 4 to give exclusively 3-bromo-5-phenylisothiazole-4-carbonitrile 5. Suzuki cross-coupling conditions applied to 3-chloro-5-phenylisothiazole-4-carbonitrile 2a gave 3-phenoxy-5-phenylisothiazole-4-carbonitrile 6, which was prepared independently, and not the 3-phenyl derivative. All isothiazole products were fully characterized.