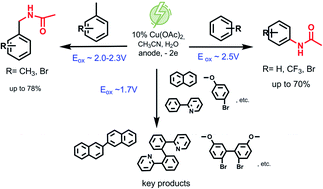
A mild, efficient electrochemical approach to the site-selective direct C–H amidation of benzene and its derivatives with acetonitrile and benzonitrile has been developed. It has been shown that joint electrochemical oxidation of various arenes in the presence of a copper salt as a catalyst and nitriles leads to the formation of N-phenylacetamide from benzene and N-benzylacetamides from benzyl derivatives (up to 78% yield). A favorable feature of the process is mild conditions (room temperature, ambient pressure, no strong oxidants) that meet the criteria of green chemistry.