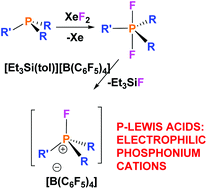
A series of fluorophosphonium salts, [R3PF][X] (R = alkyl or aryl; X = FB(C6F5)3, [B(C6F5)4]), have been prepared by reactions of phosphine/borane frustrated Lewis pairs (FLPs) with XeF2 or difluorophosphoranes with [Et3Si][B(C6F5)4]. As the substituents bound to phosphorus become increasingly electron withdrawing, the corresponding fluorophosphonium salts are shown to be increasingly Lewis acidic. Calculations were also performed to determine the relative fluoride ion affinities (FIA) of these fluorophosphonium cations.