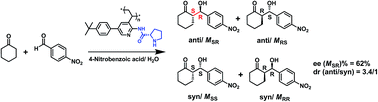
Novel optically active polymers containing pendant proline moieties, poly{(−)-L- and (+)-D-proline-[5-(4′-tert-butylphenyl)-3-vinylpyridin-2-yl]amide}, were prepared through radical polymerization of (−)-L- and (+)-D-N-Boc-proline-[5-(4′-tert-butylphenyl)-3-vinylpyridin-2-yl]amide, followed by de-protection of the Boc group. Polarimetry and circular dichroism spectroscopy studies indicated that these polymers took chiral secondary structures. They were employed to catalyze the homogeneous asymmetric aldol reaction of 4-nitrobenzaldehyde with cyclohexanone. The polymeric catalysts showed obviously enhanced reaction rate but slightly reduced enantio-selectivity compared with (−)-L- and (+)-D-proline-[5-(4′-tert-butylphenyl)-3-vinylpyridin-2-yl]amide, the low molar mass counterparts. The lowered enantio-selectivity of the polymeric catalyst might be attributed to the antagonistic action of the configurational chirality of the side-group and the conformational chirality of the polymer backbone on the stereochemistry of aldol reaction.