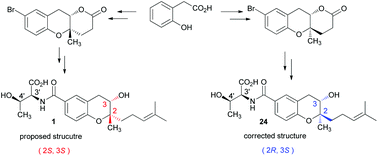
The relative and absolute configurations of xiamenmycin A, a benzopyran compound isolated from Streptomyces xiamenensis 318 with a highly potent anti-fibrotic activity, have been characterized through the total synthesis. The key steps include the construction of the 3-chromanol moiety via Sharpless epoxidation followed by regio- and diastereo-selective cyclization and introduction of the threonine moiety at a later stage via Pd-catalysed aminocarbonylation in a one-pot procedure. The stereochemical assignment of natural xiamenmycin A has been accordingly revised to be 2R, 3S, 3′S, 4′R.