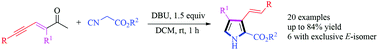A mild, transition-metal-free and facile C3-alkenylated 2,3,4-trisubstituted pyrrole cyclization of methylene isocyanides with ene–yne–ketones in moderate to good yields was explored. The E-alkenylated products were isolated in moderate to exclusive selectivity in most cases. The investigated compounds in this work are expected to open up for use as potential medicinal agents or precursors for post-modification.