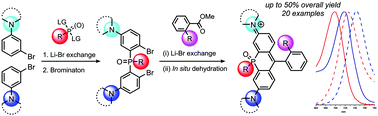
P-Rhodamines were accessed by implementing a robust three step sequence consisting of (i) addition of m-metallated anilines to dichlorophosphine oxides, (ii) selective dibromination, and (iii) cyclization of the diaryllithium reagents derived from the dibromides to form the dihydroacridophosphine core of P-rhodamines. A modified route was developed to produce non-symmetric P-rhodamines. A library of prepared P-rhodamines provides first insight into dependence of fluorophore properties on the structure of P-rhodamines. A P-rhodamine with highest batochromic shifts and quantum yields in the class was identified.