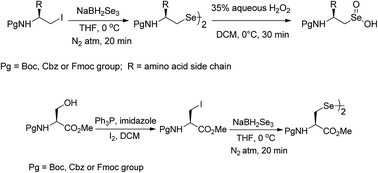
A convenient approach has been presented for the synthesis of Nβ-protected amino diselenides from the corresponding amino alkyl iodides using in situ generated NaBH2Se3 as an efficient selenating reagent. All the diselenides are obtained in good yields under very mild conditions, short duration times and the protocol is free from racemization. The methodology has been effectively extended to the synthesis of N-protected L-selenocystine methyl ester. Clean oxidation of Nβ-protected amino diselenides to Nβ-protected amino seleninic acids using 35% aqueous H2O2 has also been accomplished. The present protocol is compatible with all the common urethane protecting groups.