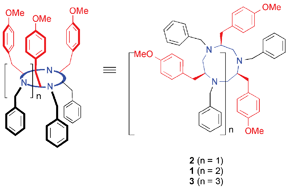
Synthesis and structure elucidation of optically active tri-, tetra-, and penta-azamacrocycles having 4-methoxyphenyl pendants are described. Regioselective ring opening of a nosylaziridine with secondary benzyl amines was repeatedly performed to afford the cyclization precursors. Intramolecular N-alkylation of N-(ω-haloalkyl) nosylamide provided tri-, tetra-, and penta-azamacrocycles. On the basis of our study of the tetra-azamacrocycle previously elucidated by X-ray single-crystal analysis and in solution by NMR analysis, we conclude that the tri-azamacrocycle does not mainly have a vase-type conformation because of the steric hindrance of the 4-methoxyphenyl groups but the penta-azamacrocycle has a vase-type conformation in CDCl3 and in CD2Cl2. The vase-type conformation of the penta-azamacrocycle is, however, not as much stable as that observed in the tetra-azamacrocycle because conformational flexibility of the penta-azamacrocycle was observed in deuterated benzene.