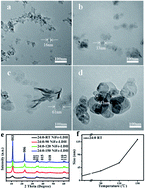
The Ni–Fe layered double hydroxide (LDH) is regarded one of the best catalysts for the oxygen evolution reaction (OER), yet bridging the relationship between the LDH nanostructure and OER performance still remains a big challenge. Instead of using other hydrothermal reactions to produce Ni–Fe layered double hydroxides, we adopted a method using a simple separate nucleation and aging steps to investigate the effect of crystallinity and the intercalated anions of LDH on OER performance. We found that improving the crystallinity and the size of NiFe-LDH by increasing the aging temperature led to a decrease of OER activity. Changing the interlayer spacing of LDH from 8.04 Å to 7.69 Å by introducing more CO32− to replace NO3− causes the reduction of OER activity. These are probably attributed to the more exposed active sites, lower charger transferring resistance, and better exchange ability with OH− in interlamination. Based on the abovementioned observations and the consequent optimizations, a very-low onset overpotential (∼240 mV) and Tafel slope value (33.6 mV dec−1) (in 0.1 mol L−1 KOH) for room-temperature synthetic NiFe LDH were achieved. This work proposes a strategy for the rational design of LDHs for the further enhancement of OER electrochemical activity, i.e. by decreasing the size and crystallinity of NiFe-LDH and by introducing more NO3− between layers.