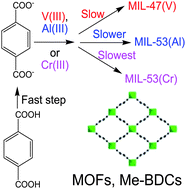
A few porous isostructural metal-benzenedicarboxylates (Me-BDCs) such as MIL-47(V), MIL-53(Al) and MIL-53(Cr) have been synthesized hydrothermally from metal chlorides, TPA (terephthalic acid) and water to elucidate the relative rates (both in the nucleation stage and crystal growth stage) depending on the metal ions. The synthesis rates are rMIL-47(V) > rMIL-53(Al) > rMIL-53(Cr) for both in the nucleation and crystal growth stages at the same temperature, illustrating the importance of lability of the metal ions in the synthesis rate of a MOF material. The lability of metal ions is important in the kinetics of MOF synthesis because the synthesis may be carried out by simple complexation or ligand exchange. The importance of the lability of metal ions may suggest that the deprotonation rate of TPA (to BDC), even in acidic conditions, is relatively fast compared with the complexation rate of BDC on metal ions to form MOFs.