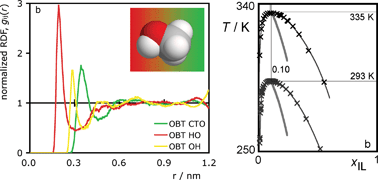
In this work, we explore the mutual solubility of a number of mixtures of commonly used ionic liquids (imidazolium, pyridinium, phosphonium and ammonium ionic liquids) with partially fluorinated n-alcohols (C7 to C10) or perfluoroheptane. The corresponding T–x diagrams at atmospheric pressure were measured through cloud-point temperature determinations. For some selected systems under near-critical isopleth conditions, pressure effects were also studied. The results are discussed in terms of (i) shifts in the immiscibility envelopes as the cation alkyl-chain length is changed, (ii) the nature of the cation or the anion, (iii) the increasing length of the fluorinated/alkylic moiety of the partially fluorinated alcohol, or (iv) comparisons with similar systems involving normal alcohols.