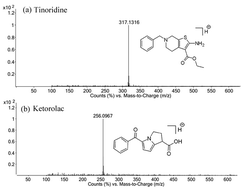
A selective, sensitive and fast liquid chromatography coupled with mass spectrometry method (LC-MS) has been developed and validated for the quantification of tinoridine hydrochloride in rat plasma using ketorolac tromethamine as an internal standard. Pre-treatment of the sample obtained from the plasma involved a simple and reliable protein precipitation method using methanol. A successful chromatographic separation was achieved on a Zorbax extended C18 column (50 mm × 2.1 mm, 1.8 μm) using acetonitrile and 0.1% formic acid as the mobile phase in gradient elution mode with a flow rate of 0.5 mL min−1. Detection was performed with positive ion electrospray ionization mass spectrometry using target ions of [M + H]+ at m/z 317 for tinoridine and m/z 256 for ketorolac in selective ion mode. The method was validated in the calibration range of 2–200 ng mL−1. All the validation parameters were well within the limits. This method was further successfully applied in pharmacokinetic studies of the drug in rats.