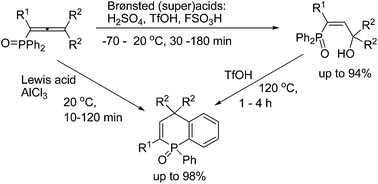
1-(Diphenylphosphoryl)alka-1,2-dienes (phosphonoallenes) in Brønsted (super)acids (TfOH, FSO3H, and H2SO4) at −70 to 120 °C for 30 min to 4 h gave, at first, (3-hydroxyalk-1-en-1-yl)diphenylphosphine oxides, as kinetically favorable reaction products, that are further converted into 1-phenyl-1,4-dihydrophosphinoline 1-oxides as thermodynamically stable compounds. The latter compounds are formed from phosphonoallenes under the action of a strong Lewis acid AlCl3 at room temperature for 10–120 min. This is a novel, simple and efficient (short reaction time, high yields) method for synthesis of such 1,4-dihydrophosphinoline 1-oxides.