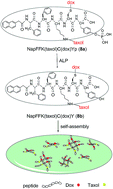
Doxorubicin (Dox) and Taxol can be covalently bonded to the same peptide segment via proper structural modification. Doxorubicin-maleimide derivative links to peptide via Michael addition reaction and Taxol-Succi-NHS active ester connects to the same peptide backbone through ester–amide exchange reaction. Enzymatic transformation, as an inherent biological process, is applied here to trigger the formation of nanofiber networks from the as prepared hydrogelator precursor. The precursor which loads equal molar ratio of Dox and Taxol can self-assemble to form a red stable hydrogel after dephosphorylation reaction catalyzed by alkaline phosphatase (ALP). This hydrogel could maintain sustained release of drugs and show strong anticancer effect. This work, as a new strategy to build a co-delivery system of covalently linked Dox and Taxol, owns the potential to serve as an injectable hydrogel for therapeutic applications.