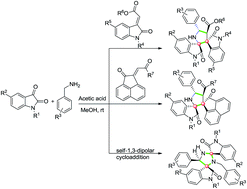
We report acetic acid promoted in situ generation of ketimine and a 1,3-dipole that results in regio- and diastereoselective formation of dispiropyrrolidineoxindoles and dispiroimidazolidineoxindoles via tandem cycloaddition. This novel one-pot reaction generates four chiral centres with two contiguous spirostereocenters in dispiropyrrolidineoxindoles, and three chiral centres with two distal spirostereocenters in dispiroimidazolidineoxindoles.