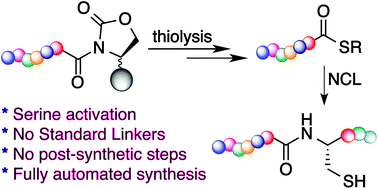
Fmoc solid phase peptide synthesis of thioesters for the chemical synthesis of proteins via native chemical ligation is a challenge. We have developed a versatile approach for direct synthesis of peptide thioesters from a solid support utilizing Fmoc chemistry. Peptide thioester synthesis is performed by the formation of a cyclic urethane moiety via a selective reaction of the backbone amide chain with the side group of serine. The activated cyclic urethane moiety undergoes displacement by a thiol to generate the thioester directly from the solid support. Importantly, the method activates the serine residue for the synthesis of peptide thioesters; thus it is fully automated and free of the types of resins, linkers, handles, and unnatural amino acids typically needed for the synthesis of peptide thioesters using Fmoc chemistry. The resulting thioester is free of epimerization and is successfully applied for the synthesis of longer peptides using NCL.