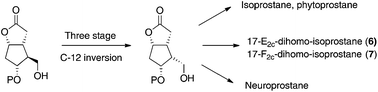
A practical methodology for the synthesis of key intermediates for isoprostane, neuroprostane and dihomo-isoprostane preparation has been described. The key strategy involved a three stage C-12 stereocenter inversion of the configuration of a Corey lactone, commercially available in an enantiopure form. The key intermediate was then used to prepare 17-E2c-dihomo-isoprostane and 17-F2c-dihomo-isoprostane.