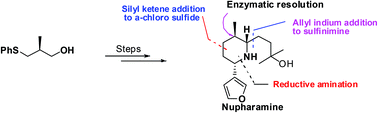
An efficient stereoselective synthesis of the nuphar alkaloid, (−)-nupharamine, is reported. The key features include the Lewis acid catalyzed reaction of an α-chlorosulfide with a silyl ketene acetal for C–C bond formation, creation of the stereocenter at C2 by a diastereoselective reaction of allyl indium with a sulfinimine and reductive amination for the introduction of the C6 stereocenter of the piperidine ring.