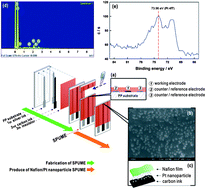
We report here a highly selective hydroxyl radical assay through the depletion of H2O2 as an indicator for iron-catalysed hydroxyl radical formation without any separation process and chemical probe. By purging with N2 gas under suitable humidity, H2O2 can be carried out with water and detected in the gas media using a disposable sensor made of a screen-printed edge band carbon ultramicroelectrode deposited with Pt nanoparticles and coated with Nafion as the solid polymer electrolyte. This H2O2 detection scheme is highly selective and thus can monitor the hydroxyl radical without any separation process and chemical probe. Based on Fenton chemistry, the quantitation of the hydroxyl radical is achieved through the suppressed peak current of hydrogen peroxide with a linear dependence on [Fe2+] in the range of 25 μM to 1 mM and a detection limit of 4.48 μM (S/N = 3). The proposed method has been applied to evaluate the antioxidative activity of phenolic compounds and catechins.