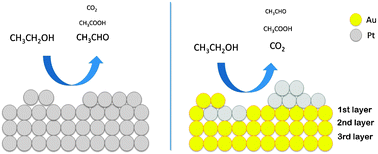
The ethanol electro-oxidation reaction was evaluated using a polycrystalline Au substrate modified with two different amounts of Pt using the galvanic exchange methodology. FTIR results suggest that Pt deposits have a greater ability to break the C–C bond present in the ethanol molecule. However, under potentiostatic conditions both modified Au surfaces undergo faster deactivation in comparison with polycrystalline platinum as indicated by the chronoamperometric results. XPS results indicate the presence of two phases depending on the Pt content. These are: (i) Pt–Au alloy and (ii) segregated Pt. The structural and electronic properties of these phases were related to the differences observed in the catalytic activity.