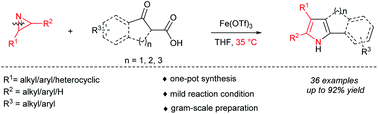
An Fe(III)-catalyzed Mannich/decarboxylative ring-expansion reaction for the synthesis of pyrrole derivatives is reported. β-Ketoacids were employed as enolate equivalents of a simple ketone and were coupled with 2H-azirine in a formal [3 + 2] cycloaddition reaction. A series of di-, tri-alkyl/aryl-substituted and fused pyrrole derivatives was obtained in moderate to excellent yields (17–92%) under mild reaction conditions. The products could be synthesized on gram-scale with 5 mol% catalyst loading.