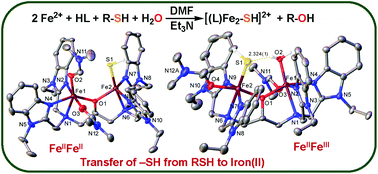
While the attempted synthesis of diiron(II)–hydrosulfide complexes using HS− produced an insoluble precipitate, the reaction of Fe(BF4)2·6H2O, Et3N and HN-Et-HPTB with RSH (R = tBu, CH2Ph) yielded the desired complex, [Fe2(N-Et-HPTB)(SH)(H2O)](BF4)2 (1a). The synthesis, one electron oxidation and dioxygen activity of 1a in comparison with an analogous chloride complex, [Fe2(N-Et-HPTB)(Cl)(DMF)2](BF4)2 (2), are described.