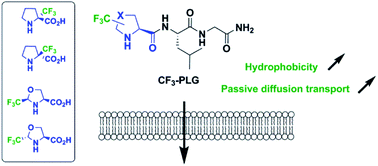
The synthesis of four CF3-proline analogues of the PLG peptide is reported. Our results show that the incorporation of trifluoromethylated amino acids (Tfm-AAs) at the N-terminal position of a peptide significantly increases its hydrophobicity. In addition, depending on the relative configuration and the position of the CF3 group, Tfm-AAs can also promote passive diffusion transport.