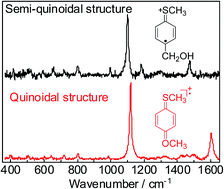
To understand dimerization-reactivity, structures of 4-substituted thioanisole radical cations (ArSCH3˙+) in aqueous solution were studied by the nanosecond time-resolved resonance Raman (ns-TR3) spectroscopy during pulse radiolysis and density functional theory (DFT) calculations. The downshift of the C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C stretching mode of the benzene ring and the upshift of the C–S stretching mode upon oxidation were observed with aromatic sulfides such as 4-hydroxymethylthioanisole (MTPM), suggesting formation of a semi-quinoidal structure. For the 4-methoxythioanisole radical cation (MTA˙+), weak CPh–O stretching was observed additionally. Based on the DFT calculations, semi-quinoidal structures including an S atom and benzene ring were indicated for MTPM˙+ and so on, in which the positive charge and spin are localized on the S-atom and C4-position, respectively. On the other hand, the quinoidal structure including the S and O atoms and benzene ring was indicated for MTA˙+. The results of DFT calculation agreed well with TR3 spectra of ArSCH3˙+. The reactivities of ArSCH3˙+ toward dimerization were discussed in terms of the conjugation between S non-bonding electrons and π-electrons of the aromatic system.
C stretching mode of the benzene ring and the upshift of the C–S stretching mode upon oxidation were observed with aromatic sulfides such as 4-hydroxymethylthioanisole (MTPM), suggesting formation of a semi-quinoidal structure. For the 4-methoxythioanisole radical cation (MTA˙+), weak CPh–O stretching was observed additionally. Based on the DFT calculations, semi-quinoidal structures including an S atom and benzene ring were indicated for MTPM˙+ and so on, in which the positive charge and spin are localized on the S-atom and C4-position, respectively. On the other hand, the quinoidal structure including the S and O atoms and benzene ring was indicated for MTA˙+. The results of DFT calculation agreed well with TR3 spectra of ArSCH3˙+. The reactivities of ArSCH3˙+ toward dimerization were discussed in terms of the conjugation between S non-bonding electrons and π-electrons of the aromatic system.