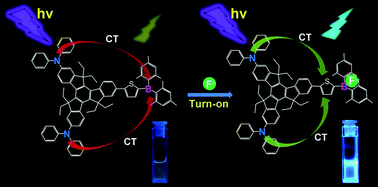
Fluoride anion (F−) significantly affects chemical, biological, and environmental processes. Fluoride recognition and detection have received increasing attention. Convenient, effective, and sensitive fluorescent probes for F− should urgently be designed and synthesized. In this study, we describe a strategy for constructing two triarylborane-based fluoride fluorescent probes: 2,7,12-tri(2-(5-(dimesitylboryl)thiophen-2-yl)ethynyl)-5,5′,10,10′,15,15′-hexaethyltruxene (C3B3) with π–3A (acceptor) configuration and 2,7-di(N,N-diphenylamino)-12-(5-(dimesitylboryl)thiophen-2-yl)-5,5′,10,10′,15,15′-hexaethyltruxene (N2SB) with 2D (donor)–π–A configuration. The loss of color of the tetrahydrofuran solution of these probes from greenish yellow suggests that they can conveniently monitor F− at a low concentration (10 μM) free of apparatus. The different structural features of these probes varied their fluorescent responses to F−. The single-photon fluorescence intensity of C3B3 declined to 90% upon the addition of 4.5 equivalents of F− to its tetrahydrofuran solution. However, the single-photon fluorescence intensity of N2SB was enhanced six-fold upon addition of 2.5 equivalents of the F−. Under the experimental conditions, the detection limits of the two probes for F− can reach 12–13 μM (C3B3) and 3–5 μM (N2SB). The ability of the two probes in detecting F− in their toluene solutions in the two-photon mode was also investigated. The sensitive two-photon fluorescence responses of both probes make them excellent two-photon fluorescence probes.