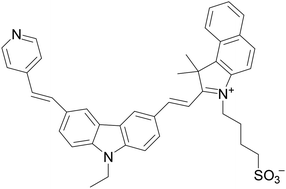
Many organic ligands were synthesized to recognize G-quadruplexes. However, different kinds of G-quadruplexes (G4s) possess different structures and functions. Therefore, selective recognition of certain types of G4s is important for the study of G4s. In this paper, a novel cyanine dye, 3-(2-(4-vinylpyridine))-6-(2-((1-(4-sulfobutyl))-3,3-dimethyl-2-vinylbenz[e]indole)-9-ethyl-carbazole (9E PBIC), composed of benzindole and carbazole was designed and synthesised. The studies on UV-vis and fluorescence properties of the dye with different DNA forms showed that the dye exhibits almost no fluorescence under aqueous buffer conditions, but it increased over 100 fold in the presence of c-myc G4 and 10–30 fold in the presence of other G4s, while little in the presence of single/double-stranded DNA, indicating that it has excellent selectivity to c-myc 2345 G4. For the binding studies the dye is interacted with the c-myc 2345 G-quadruplex by using the end-stack binding model. It can be said that the dye is an excellent targeting fluorescent probe for c-myc G-quadruplexes.