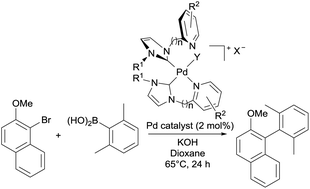
A family of electronically diverse pyridyl- and picolyl-substituted imidazolium salts have been prepared and coordinated to palladium in a single step, to deliver a variety of palladium(II)–N-heterocyclic carbene (NHC) complexes. Neutral Pd(NHC)X2, cationic [Pd(NHC)2X]X and dicationic [Pd(NHC)2]X2-type complexes have been isolated and fully characterised, with single-crystal X-ray analysis revealing a variety of coordination environments around the palladium centres. The pre-formed complexes have been employed in a model Suzuki–Miyaura cross-coupling reaction to yield a sterically congested tetra-ortho-substituted biaryl product, showcasing turnover numbers comparable to Pd-PEPPSI-IPr catalyst.