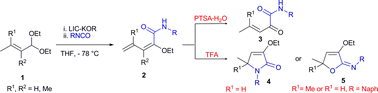
Alkoxydienamides 2 have been synthesized exploiting the reactivity of α,β-unsaturated acetals 1 with isocyanates in the presence of Schlosser's superbase LIC–KOR. In a mild acidic medium, 2 can then be promptly converted both into α-ketoamides 3 and into substituted 2-pyrrolidinones 4 or imino ethers 5 by choosing the appropriate experimental conditions.