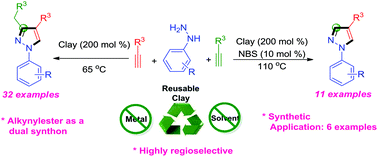
A highly regioselective, solvent-free and montmorillonite K10 clay-catalyzed domino process with an unprecedented ring-closing C–C bond-formation reaction is described for the synthesis of a new class of 1,3,4-trisubstituted and 1,4-disubstituted pyrazole derivatives. The reaction proceeded via nucleophilic attack of enamine from an intermediate on the β-carbon of an amino acrylate. Experimental outcomes clearly revealed that organic solvents had an adverse effect upon pyrazole formation, and that an oxidative condition in the presence of the reusable clay favored formation of the 1,3,4-trisubstituted pyrazole. The clay in the presence of a N-bromosuccinamide catalyst gave the 1,4-disubstituted pyrazole after pyrolytic cleavage at elevated temperature.