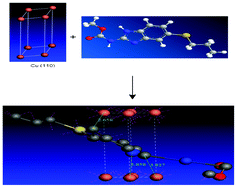
The interaction and corrosion inhibition properties of methyl carbamate on copper metal in 1 N HNO3 have been studied by polarization, electrochemical impedance spectroscopy (EIS), adsorption, surface studies and computational calculations at 300 K. Polarization studies showed that methyl carbamate act as a cathodic type inhibitor. The inhibitive action of this molecule is discussed in terms of blocking the electrode surface by the adsorption of inhibitor molecules obeying Langmuir isotherm. In addition, the local reactivity, analyzed through Fukui functions, shows that the sulphur atom will be the most probable adsorption site. A quantum chemical approach is used to calculate electronic properties of the molecule to ascertain the relation between inhibitive effect and molecular structure.