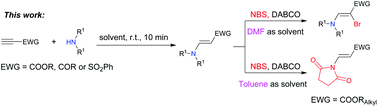
A new two-step one-pot aminobromination/chlorination of carbonyl alkynes has been achieved via a Michael addition of aliphatic secondary amines and subsequent β-bromination/chlorination of the obtained enamines to afford various α-X (X = Br or Cl) enamino ketones/esters in moderate to good yields. A solvent-controllable protocol has been developed to produce versatile 3-(2,5-dioxopyrrolidin-1-yl)acrylates in moderate yields by using toluene as the solvent and chain alkyl propiolates as alkynyl substrates.