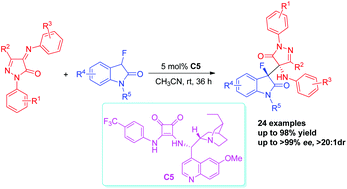
An enantioselective Mannich reaction between 3-fluorooxindoles and pyrazolinone ketimines has been developed for the construction of amino-pyrazolone-oxindoles containing stereogenic C–F units. Based on this new protocol that allows for the generation of two adjacent tetrasubstituted stereocenters, a variety of structurally diverse fluorinated amino-pyrazolone-oxindoles were obtained in good to excellent yields with excellent diastereoselectivities and enantioselectivities (up to 98% yield, >20 : 1 dr and >99% ee). What's more, good yield and high stereoselectivities were obtained in the gram-scale reaction.