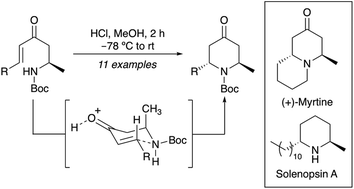
An acid-mediated 6-endo-trig cyclisation of amine-substituted enones has been developed for the stereoselective synthesis of trans-6-alkyl-2-methyl-4-oxopiperidines. Performed under conditions that prevent removal of the Boc-protecting group or acetal formation, the key cyclisation was found to generate cleanly the 4-oxopiperidine products in high overall yields from a wide range of alkyl substituted enones. The synthetic utility of the trans-6-alkyl-2-methyl-4-oxopiperidines formed from this process was demonstrated with the total synthesis of the quinolizidine alkaloid, (+)-myrtine and the piperidine alkaloid, (−)-solenopsin A.