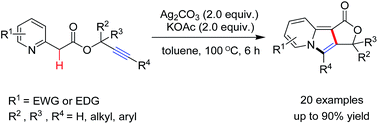
The treatment of a toluene solution of easily accessible 2-(pyridin-2-yl)acetic acid propargyl esters with 2.0 equiv. of Ag2CO3 in the presence of potassium acetate (2.0 equiv.) at 100 °C afforded fused tricyclic indolizines in good to excellent yields. The reaction proceeded through a domino silver-mediated double cyclization sequence involving a 5-exo-dig cyclization and 1,3-hydrogen shift followed by an intramolecular cycloisomerization.