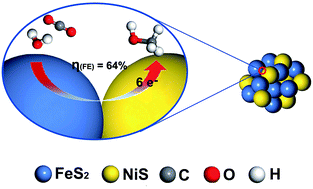
Electrochemical reduction of carbon dioxide (CO2) to methanol (CH3OH) catalyzed by transition metals has been proved feasible and effective in aqueous electrolytes. In this work, we introduce a FeS2/NiS nanocomposite electrocatalyst synthesized by traditional hydrothermal method, which selectively reduces CO2 to CH3OH with an unprecedented overpotential of 280 mV and a high faradaic efficiency up to 64% at the potential of −0.6 V vs. reversible hydrogen electrode (RHE). The FeS2/NiS nanocomposite electrocatalyst exhibits a stable current density of 3.1 mA cm−2 over a 4 hour stability test. The high selectivity towards CO2 electroreduction to CH3OH may be attributed to the special ladder structure of the FeS2/NiS nanocomposite. The active sites are located at the interface between FeS2 and NiS which can effectively suppress the side reaction hydrogen evolution reaction and facilitate the CO2 reduction reaction.