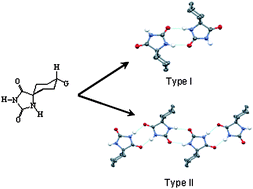
The preparation of 8-methoxy-2,4-dioxo-1,3-diazaspiro[4.5]decane (1), 8-butoxy-2,4-dioxo-1,3-diazaspiro[4.5]decane (2), ethyl-2,4dioxo-1,3-diazaspiro[4.5]decane-8-carboxylate (3), 8-tert-butyl-2,4-dioxo-1,3-diazaspiro[4.5]decane (4) is described. The relationship between the molecular structure and crystal structure of 1, 3, 4 and 2,4-dioxo-1,3-diazaspiro[4.5]decane-8-carboxylic acid (5) is discussed. Crystallographic analysis shows that the crystals do not contain solvent molecules and highlights the role that the substituents on the cyclohexane ring play in supramolecular arrangements. Although the R22 (8) (CONH)2 hydrogen bond ring is generally retained, two types of structures have been defined as a function of the interactions between hydantoin rings, with Type I and Type II formed by dimers and ribbons, respectively.