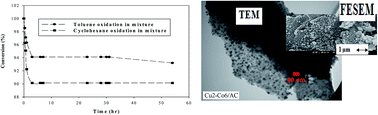
Copper and cobalt oxides supported on almond shell derived activated carbon (AC) with different loadings were synthesized by sequential and co-deposition–precipitation methods leading to Cu(shell)/Co(core)/AC, Co(shell)/Cu(core)/AC and Cu–Co(mixed)/AC catalysts that were subsequently used for catalytic oxidation of gaseous mixtures of toluene and cyclohexane in air in a tubular flow reactor. The catalysts and the support were characterized by Boehm test, Brunauer–Emmett–Teller (BET) surface area, X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDX), inductively coupled plasma (ICP), and thermogravimetric analysis (TGA). Catalyst efficiency for toluene and cyclohexane oxidation, both separately and in a mixture, was higher over the mixed metal oxides catalysts compared with the core–shell catalysts. An increase in the cobalt loading on the support led to a decrease in the metal oxide crystallite size and a change in the catalyst morphology. The best performance was obtained for the Cu2–Co6(mixed)/AC sample (Removal Efficiency >99.9%). Agglomeration of copper oxide over cobalt oxide crystallites for Cu(shell)/Co(core)/AC samples resulted in catalysts with the worst performance for complete oxidation of VOCs. Results indicated a negligible deterrence effect of toluene on cyclohexane oxidation. Furthermore, the addition of water (humid air) decreased the conversion of hydrocarbons.